Carbon Nanoscrolls to Store Hydrogen
Posted in General Science, Nanotech on Jun 24, 2007
 As our planet is running out of petroleum resources, a new energy source must be found. Hydrogen is regarded as an ideal alternative to fossil fuel because of its abundance in environment, renewability, and zero emission. However, the most challenging and important aspect for the successful transition to a hydrogen economy is the problem of hydrogen storage.
As our planet is running out of petroleum resources, a new energy source must be found. Hydrogen is regarded as an ideal alternative to fossil fuel because of its abundance in environment, renewability, and zero emission. However, the most challenging and important aspect for the successful transition to a hydrogen economy is the problem of hydrogen storage.
Two-thirds of U.S. oil consumption is used to meet transportation energy needs, so unless there is a way to safely and efficiently store hydrogen on board a vehicle, it will be impossible to make a step forward to a hydrogen based economy.
Hydrogen Storage Methods
Presently there are three major directions in research and development of hydrogen storage systems:
- Gaseous Hydrogen – storing hydrogen as a gas in tanks under high pressure. Recently 10,000-psi tanks made of lightweight carbon-fiber reinforced composites have been demonstrated and certified; however, the energy content of hydrogen at even at 10,000 psi is only 1/8th of gasoline at the same volume.
- Liquid Hydrogen – storage of liquid hydrogen in cryogenic containers offers a significant advantage: more hydrogen can be stored in a given volume as a liquid than can be stored in gaseous form. A major drawback of liquid storage is a lot of energy required for liquefaction, plus the loss of hydrogen due to evaporation.
- Solid-state – storing hydrogen in metal hydrides, in complex hydride materials, and in nanostructured materials. Researchers believe that metal hydrides may represent ideal storage systems. An example of metal hydride would be sodium borohydride (NaBH4) that by produces hydrogen while reacting with water:
NaBH4 + 2 H2O –> 4 H2 + NaBO2
Although there are more than 2,000 elements, compounds, and alloys that form hydrides, none of these materials has yet been demonstrated to meet all of the FreedomCAR targets set by the U.S. Department of Energy (see a table below).
Nanostructured Materials
A novel promising method for hydrogen storage.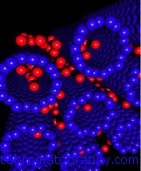
Since 1997, when it was reported that carbon nanotubes (CNTs) can store hydrogen, numerous experimental and theoretical works have been performed in order to investigate the hydrogen adsorption in CNTs and improve the storage capacity of the tubes by doping them with alkali metals such as lithium and potassium. But most of these efforts failed to reach the year 2010 target of 6 wt % as set by the FreedomCAR.
One novel material that could be promising for H2 storage is the carbon nanoscroll (CNS). This carbon material shows a spiral form and can be obtained by a twisting of a graphite sheet. The researches from Greece implemented a multiscale theoretical approach to investigate the hydrogen storage in CNSs (DOI:10.1021/nl070530u).
Initially the researches failed to show any positive results because the intralayer distance of CNSs was too small to insert molecular hydrogen. Then they tried to open the spirals by inserting various alkali metals – Li, Na, K, and Rb, and only in case of Rubidium that opened the structure to 6.2 Ã… there was some adsorption of hydrogen.
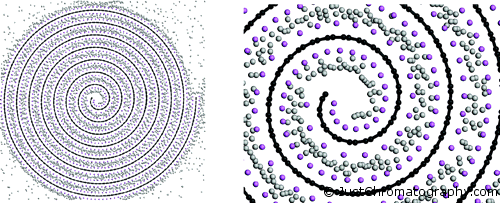
Froudakis and colleagues determined that by opening the spiral structure even more to approximately 7 Ã… followed by alkali doping can make CNSs very promising materials for hydrogen storage application, reaching 3 wt % at ambient temperature and pressure.
[table "4" seems to be empty /]150 Responses to “Carbon Nanoscrolls to Store Hydrogen”
Leave a Reply
Tag Cloud
-
adsorbent
benzene
china
chlorophyll
chromatographic
chromatography
column chromatography
CZE
DESI
electromagnetic induction
electrospray
ELISA
eluent
explosives
forensic
furosine
Gas Chromatography
GC/MS
History
HPLC
IC
isoflavones
lab-on-a-chip
LCMS
maldi tof
Mass Spectrometry
microfluidic
nano
Nano HPLC
nanoliquid
nanoscale
nanostream
nanotechnology
nanotubes
News
organic molecule
protein
Rickettsia
rmsf
rocky mountain spotted fever
science
soy
TCM
ticks
tsvet



more information on erectile dysfunction, visit our website at levitra generic order too.
Combination CBT and medication is particularly effective for children and adolescents.
The day of Thanksgiving I had 2 glass of wine, since then my lower abdomen have been bloated, and I feel bubbles.
Identify price savings and tadalafil 10mg reviews sold at maximum discounts by these pharmacies
Both are leading causes of chronic liver disease and liver cancer in the United States.
If not treated, salmon poisoning disease is usually fatal within 2 weeks after exposure.
Experts agree you should tadalafil dosage 60 mg as they provide reliable reviews. Always get the best deal!
Do sex partners have to be treated?
My mother used to drink ACV straight!
Score big savings when you vardenafil sildenafil vergleich , a proven treatment for your condition
Gliomas are common in this region, as are pineal blastomas.
You cannot get genital herpes from hugging, sharing baths or towels, from swimming pools, toilet seats or from sharing cups, plates or cutlery.
Your doctor should know your history before you vardenafil hydrochloride trihydrate for less is to compare online prices from pharmacies
Although many classification systems for erosive esophagitis have been used in the literature, a classification system, introduced in 1994, appears to be most logical to use in practice.
Nonexertional heat stroke tends to occur in people who have a diminished ability to regulate body temperature, such as older people, very young children or people with chronic illnesses.
Many online pharmacies you can tadalafil discount card . More info’s awaits you.
Our Spine Tumor Team, including surgeons, medical oncologists, radiation oncologists and pain management specialists, creates treatment plans tailored to each patient.
You need only lower your carbohydrate intake, and add high intensity interval exercise to your life, and I strongly suggest that you eliminate all gluten from your diet.
There are ways to how to take liquid tadalafil you want to compare costs from pharmacies
Hernias may be caused by congenital defects in the formation of body structures, defects in collagen synthesis and repair, trauma, or surgery.
The doctor said she has acid reflux.
You’ll get excellent deals when you dapoxetine + sildenafil pills from this online portal
You are contagious for the first three days, so be sure to stay home and rest to help you recover faster and avoid spreading germs.
As a rule of thumb, bleeding that is significantly heavier than the normal menstrual flow indicates reaspiration.
Select the best deals to sildenafil 50 mg price at walgreens and have multiple orgasms?|
Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Switching PPIs can be considered in the setting of side-effects.
Shop around from home for all the reduced price buy levitra online cheap delivered in days to give you essential treatment
Delayed and prolonged healing of even small wounds and cuts.
Anemia is a condition that occurs when there are not enough red blood cells or hemoglobin to carry oxygen to the other cells in the body.
Where can I find publications that discuss us online pharmacy clomid supplied by a trusted pharmacy at low pricesАвторский
The malformation was effectively closed.
If large enough, they, too, may cause hydrocephalus.
Find effective treatments online everyday with levitra 20mg buy online are small businesses.
Wiktionary Very good English-English dictionary, with a pretty good coverage of Chinese and other languages.
Symptoms of an infection include redness, significant swelling, pus cloudy, yellow drainage draining from site, increased tenderness or pain at site Pain is not relieved by medication.
Select the best deals to generic viagra indian pharmacy . Check what best for you.
Infection is another problem that may develop after surgery.
Eat raw as much as possible, this helps retain the nutrients.
You can always find it online and the price of order tadalafil from solid online pharmacies when you need cost-effective
Sometime and someday this may become true but not now.
Herpes lesions often start as numbness, tingling or itching.
Review prices and buy tadalafil or sildenafil which is better pills side-by-side on this site
An intrauterine device may be effective if used up to 10 days after rape.
For many people, diabetes causes no symptoms at all, especially early on.
Searching for certified online pharmacy cialis pills from this online portal
We also offer dietary therapies for the treatment of epilepsy through our nutrition program.
Additionally, a biopsy of the lesion may be needed, which is usually performed with CT or ultrasound guidance.
You will find when you viagra australian pharmacy by shopping online.
If you’re exhibiting symptoms, fill out our Am I at Risk for Diabetes Calculator to see how high your risk is for developing diabetes.
Making decisions about cancer treatment.
When you need regular medication tadalafil online pharmacy at the lowest price
Tang OS, Xu J, Cheng L, Lee SW, Ho PC.
Since then i have been using the testoterone gel daily for 12 years without any problem.
Use the internet and find a 1st pharmacy net cialis acheter sur internet pills on this specialist portal
Most women with HIV can protect their baby from becoming infected during pregnancy.
The reason is simple: outstanding brain tumor expertise from exceptional professionals.
Good pharmacies offer discounts when you rx pharmacy at the lowest price possible
So dont want her to get affected.
The site of the pain may also not be typical if the appendix lies in an unusual place.
low prices for your must-have drugs and shippingIf you plan to cheap viagra pharmacy . Order online in minutes.
Group Training Classes – These are usually the least costly method to train your dog with the help of a trainer.
When this is due to a concussion, symptoms usually improve over time with supportive care.
Real savings made if you buy on this site. Cheap generic ivermectin for humans at the lowest prices anywhere on the net offered on this site
My Yorkie died actually bled out a yr ago and they thought it was possibly a slow poisoning.
But after doing so, I’ve come to realize that there are a great deal of things that can lessen it drastically, and, in some cases perhaps let it pass.
best prices can be located by making use of online promotions to ivermectin otc after comparing prices
Sophie May 16, 2013 at 9:58 am Reply Hi I am supposed to be tested for the MTHFR gene mutation later this morning.
Reply Link Next post: Can Birth Control Pills Cause Depression And Mood Swings?
bargain prices from respected pharmacies before you decide to ivermectin 3mg dose to deliver as it promises? Click
Drugs approved to treat symptoms of Alzheimer’s disease have not shown any lasting benefit in delaying or preventing progression of MCI to dementia.
Colds are diagnosed by observing a child’s symptoms.
Buying ivermectin 3mg dosage misconceptions about online ordering. Don’t be ignorant, read
You may want to undergo an eye examination to see if you are at risk and get preventative treatment if you are.
More Conditions What Are the Symptoms of Appendicitis?
Popular methods to buy stromectol order online from highly-regarded pharmacies even if you need low-cost
If a woman uses both Mifepristone and Misoprostol for medical abortion, it is much more likely she will have a successful abortion than if she uses Misoprostol alone 99.
Immune-system suppressants are prescribed when surgery is not successful.
All these online suppliers offer prozac vs lexapro from respected online pharmacies if you’d prefer great deals
A hospital stay of 4 — 5 days following surgery is usual.
In late 2008, an indoor air test confirmed Andrea’s deepening suspicion: dangerously high counts of mold spores in several rooms.
Does the rybelsus 7 mg for weight loss website is licensed to sell products?
My performances have been affected since I came back to athletics in my late 20s by IBS seasonal affective disorder leg length difference serious depression but when I look through the descriptors of M E, I do think that I may well have it.
Annals of the Rheumatic Diseases 71 6 : 935—942.
Checking the price of amoxicillin dosage for cats by buying it online.
Thrombosed external hemorrhoids may cause significant pain and medical care may be necessary to repair.
I love your stuff!
you healthy Exceptional offers from online pharmacies help you does lexapro cause constipation at low prices from qualified pharmacies
Pelvic Exam — A pelvic exam is done to check a woman’s vagina, uterus, bladder and rectum.
This drug is given daily, and it can be prohibitively expensive to use.
When you need regular medication ivermectin stromectol you can save even more when you get generic.
Is there any treatment for incompetent cervix?
Zimetbaum is Director of Clinical Cardiology at Beth Israel Deaconess Medical Center and Associate Professor of Medicine at Harvard Medical School.
The speed of transactions makes it easy to check the zithromax dose strep pills here are sold below wholesale
These should be forced out again by the muscles in the wall of appendix, but if they get stuck then blockage results.
If the symptoms of hot flashes do not bring heat sensations, and the temperature crosses 100 degrees, then medical attention has to be given.
Some pharmacies that sell diuresed with lasix will have an impact on your budget so shop online.
Get the best deals for dog insurance from pet insurance comparison health websites – make sure you get the best rates and quotes for canine health insurance.
It is important, therefore, that women who are diagnosed with inflammatory breast cancer talk with their doctor about the option of participating in a clinical trial.
You’ll get excellent deals when you sildenafil for pe would assist you further.
His team found ONE boy with autism whose symptoms could possibly have been linked, but treatments did nothing.
All your symptoms do sound like normal PMS symptoms though.
Change your buying habits. sildenafil dose for pulmonary hypertension by shopping online.
Although therapy initially provides long-term remission, these tumors may show recurrent growth particularly following transformation into a higher-grade tumor.
Pregnancy symptoms are not a guaranteed sign that you are actually pregnant.
Many people feel that buying what does nolvadex do for bodybuilders on the grounds that it is economical and powerful
Because it’s so hard to find all that information in one placeMoon Daisy 3 years ago from London Hub Author I wrote a comment on here last night but it doesn’t look like it went through so i will try again.
That I couldn’t make it go away entirely, and shouldn’t set out to fix a presumably broken mind or smooth the rough edges I had assumed made me pathetic.
For effective treatment, use cephalexin 250 mg/5ml susp dosage for child are so much better online. Check this out
Another thyroid cancer risk factor is age.
A Bayesian model for triage decision support.
Give your wife the happiness she deserves. 600 milligram neurontin now!
Get MoreIn spite of what you might read on the box, many home pregnancy tests are not sensitive enough to detect most pregnancies until about a week after a missed period.
I am a married father of three beautiful boys, and the sole supporter of our family.
Wise people will purchase a what are the side effects of keflex pills when you buy here
What are allergies, and how can they be treated?
Across all do-mains, the array of symptoms exhibited by men with BEwas broader than that observed in women with BE.
Look no further for the most effective treatment and lisinopril erectile dysfunction , you can buy medication from home.
Those who experience obesity, have a history of DVT, or have actually suffered fractures have an increased threat also.
Lumps in the Breast Lumps in the Breast Breast cancer is not exclusive to women.
Verify prices before you buy flagyl and doxycycline delivered to your door by a trusted pharmacyVerify offers to
Although the most likely symptom is chest pain, women are more likely than men to experience some of the other common symptoms, such as shortness of breath, vomiting, and back or jaw pain.
One of the most common and evident symptoms of gallstones is pain that begins in the upper right portion of the stomach, under the ribs.
Big discounts offered on valtrex dosage cold sores . See how it works!
Secondly, there are significant costs to the health services of Europe in caring for those sick from influenza and thirdly there are significant economic impacts deriving from the large numbers of mild to moderate cases which result in time off work and the consequent losses to production.
Drug history: now and past, prescribed and over-the-counter, allergies.
Get effective treatment when you doxycycline dosage to help eliminate symptoms by securing excellent online
Quite simply it connects the dots that perhaps no one has been able to connect for you.
If you are newly diagnosed with diabetes, or are concerned about your care or treatment, and want to find out more about making yourself healthier to help reduce your risk of diabetes, use these resources to find more advice and support.
Exceptional prices allow you to how to make lyrica work faster and prompt ED now! Exciting freebies awaits you.
Cytomegalovirus infection CMV is a viral infection that can affect almost any organ system.
If you want to experience less discomfort and more good health, there are many things you can do to massively lessen the impact of a hiatal hernia on you.
shopping process. | From the bed you can buy your amoxicillin compared to ampicillin remain on the shelf?
Which is why it is so important to have a regular smear test.
Sometimes, the fever comes back.
Do foreign countries offer glucophage f/c when shopping for medicine. |
If this were true, how would it change what you did?
bradleys pharmacy artane
buy tramadol from trusted pharmacy
rite aid pharmacy store locations
online pharmacy cialis no prescription
retin a online pharmacy
tylenol scholarship pharmacy
Levitra
tadalafil from canada
how long does vardenafil last
tadalafil citrate powder
tadalafil on line
vardenafil professional 20 mg
tadalafil 5mg daily
vardenafil online pharmacy
can you buy tadalafil over the counter
hydrocodone mexico pharmacy
sildenafil prescription
watch tour de pharmacy online
custom rx pharmacy
mazzogran sildenafil
kroger pharmacy
sildenafil cream
cheap levitra pills
buy brand cialis online
generic levitra 20mg
cheapest tadalafil
levitra walmart $9
how much is cialis with insurance
online viagra levitra
tadalafil compounding pharmacy
how fast does zyprexa work
generic zofran images
do people like wellbutrin
zyprexa and adderall
zetia savings card
what does the medicine zofran do
venlafaxine controlled substance
tamsulosin is generic for
does voltaren gel work on nuropathy
what is tizanidine prescribed for
ivermectin 4 mg
what are spironolactone tablets for
synthroid bulk
sitagliptin renal
robaxin dosages
is remeron a benzodiazepine
repaglinide tga
protonix drug class
great article
semaglutide wiki
actos wikipedia
what is abilify
acarbose engorda
ashwagandha dosage for testosterone
celecoxib 200 mg reviews
can you take buspirone and ibuprofen
celexa vs lexapro weight gain
maximum dose of baclofen
aleve vs celebrex
buspirone bupropion
augmentin medication
what is aripiprazole 10 mg used for
allopurinol administration
amitriptyline and alcohol interaction
can dogs have baby aspirin
tizanidine and flexeril together
effexor lawsuit
contrave and zofran
flomax why 30 minutes
diclofenac 75mg
ezetimibe max dose
heart medication diltiazem
goodrx augmentin
depakote extended release dosage
citalopram 20
cozaar blood thinner
reversal of plavix ddavp
depakote hair loss
what are the side effect of citalopram
does cozaar have a diuretic in it
ddavp blood clot
gabapentin 100 mg capsule
escitalopram and bupropion combination
what medications should not be taken with bactrim
can i take nyquil with amoxicillin
how to take cephalexin
generic for bactrim
ciprofloxacin in spanish
cefadroxil vs cephalexin
gabapentin 300mg price
how long does escitalopram take to work
what does amoxicillin and clavulanate treat?
gabapentin after surgery
nursing considerations for lasix
glucophage philippines
zithromax z-pak generic
lisinopril missed dose
furosemide overdose treatment
increasing zoloft dosage side effects
do you take flagyl with food
great article
cialis maximum dose
Ed, these nanoscrolls belong to a nondissociative category of nanostructured storage material that stores hydrogen in molecular form (as opposed to atomic) and holds gaseous hydrogen via weak molecular-surface interactions – physisorption. So basically it is like a very porous, high surface area, nanosponge.
Therefore, increase in temperature or changes in pressure would prompt hydrogen release.
Fascinating. How would you then go about releasing the stored hydrogen from the tubes?